Sleep apnea is a serious sleep disorder that affects the breathing of millions of people, often leading to excessive daytime sleepiness and other health complications. CPAP (continuous positive airway pressure) machines are commonly prescribed to treat sleep apnea.
Unfortunately, in June 2021 Philips Respironics, one of the world’s largest CPAP manufacturers, announced a recall on certain CPAP models due to issues with polyester-based polyurethane (PE-PUR) sound abatement foam breaking down into black pieces that may enter the CPAP tube and be inhaled or swallowed by the user
In this blog post, we will bring you up to date on the Philips Respironics CPAP recall and review its impact on sleep apnea patients.
Background on the Philips Respironics CPAP Recall
On June 3rd, 2021, Philips issued a voluntary recall for specific CPAP(continuous positive airway pressure) models due to the breakdown of their polyester-based polyurethane (PE-PUR)foam which is used for sound and vibration abatement.
After a thorough investigation, Philips concluded that this PE-PUR foam was deteriorating and releasing harmful chemicals and particles into the air tube which could be inhaled or swallowed by users. Irritation of skin, eyes, nose, respiratory tract (airway), or even toxic or cancer-causing effects on organs such as kidneys and liver are possible outcomes of this exposure.
As a result, the Philips Respironics CPAP recall affected almost 690,000 CPAP devices in the United States and Canada.
Manufacturers like Philips are obligated to submit Medical Device Reports (MDRs) if they come across an incident that could point to one of their products as responsible for causing serious injury, death or malfunction. The FDA welcomes voluntary reports from health professionals, patients and consumers about any device-related adverse events and malfunctions.
Between 2011 and April 2021, Philips filed 30 MDRs with the FDA that were associated with PE-PUR foam breakdown. Of those reports, eight stemmed from the United States and none of them reported any patient injury or death. In April 2021, Philips officially informed the FDA of their intent to launch a field action due to worries regarding foam degradation in certain CPAP machines, BiPAP machines, and ventilators.
Since April 2021, the FDA has received more than 90,000 MDRs, including 260 reports of death, reportedly associated with the PE-PUR foam breakdown or suspected foam breakdown.
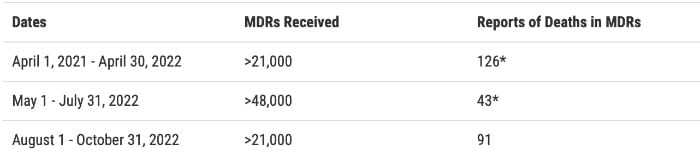
*The number of deaths has been updated to reflect Philips’ retrospective review of MDRs.
The Recall Process
Philips Respironics is doing its best to rectify this situation for CPAP users who use devices affected by the recall.
CPAP machines covered under the recall are A-Series BiPAP A30, A-Series BiPAP, A-Series BiPAP Hybrid A30, A-Series BiPAP V30 Auto (ventilator), C-Series ASV (ventilator), C-Series S/T and AVAPS, DreamStation, DreamStation ASV, DreamStation Go, DreamStation ST, AVAPS, Dorma 400, Dorma 500, E30 Garbin Plus, Aeris and Life Vent (ventilator).
Philips is offering CPAP users affected by the recall two options: returning the machine for a free replacement or receiving a refund.
In response to the recall, Philips Respironics has set up a dedicated recall website and a hotline (1-877-907-7508) for affected customers. The company has also been directly contacting customers who have been identified as owning a recalled machine.
If you own a recalled machine, you should have received a recall letter with instructions on how to proceed.
Recommendations for CPAP Users
If you own a recalled machine, your options are to either return the machine to Philips Respironics for a free repair or to receive a replacement machine at no cost.
To return the machine, you will need to fill out the form provided in the recall letter and follow the instructions for shipping the device back to the company.
If you prefer to receive a replacement machine, you can call the dedicated recall hotline to request one. Philips Respironics will cover the cost of shipping both ways.
It is important to note that the recall only affects certain models of the DreamStation and System One machines and not all of the machines sold by Philips Respironics. If you are not sure if your machine is part of the recall, you can check the recall website or call the recall hotline with your questions and concerns.
In the meantime, if you own a recalled machine, it is recommended that you continue to
use the device as directed by your healthcare provider.
If you experience any problems with your machine, such as overheating or malfunctioning, you should stop using the device and contact Philips Respironics immediately.
Overall, the recall process for the affected Philips Respironics CPAP machines has been
fairly straightforward, with the company taking proactive steps to notify affected customers and provide them with options for repairing or replacing their devices. If you own a recalled machine, it is important to follow the instructions provided in the recall letter and to stay in contact with Philips Respironics to ensure that your device is either repaired or replaced promptly.
What is Sleep Apnea?
Sleep apnea is a common sleep disorder that causes a person to stop breathing repeatedly during sleep. These breathing pauses, called apneas, can last for a few seconds to a few minutes and can occur up to hundreds of times per night.
There are three types of sleep apnea: obstructive sleep apnea, central sleep apnea, and mixed sleep apnea.
Obstructive sleep apnea (OSA) is the most common type and occurs when the muscles in the back of the throat fail to keep the airway open, despite the effort to breathe.
Central sleep apnea (CSA) occurs when the brain fails to send the proper signals to the muscles to breathe.
Mixed sleep apnea is a combination of both OSA and CSA.
Apnea scores for adults are calculated by a sleep specialist after a sleep study and are divided into three categories, which correspond to different levels of OSA severity:
- Mild Sleep Apnea: An AHI of at least five events per hour, but fewer than 15.
- Moderate Sleep Apnea: An AHI of at least 15 events per hour, but fewer than 30.
- Severe Sleep Apnea: An AHI of at least 30 events per hour.

Sleep apnea can have serious health consequences if left untreated. It can lead to high blood pressure, heart disease, stroke, and even death. It is also a major cause of daytime sleepiness and can significantly impact a person’s quality of life.
Alternative Treatments for Sleep Apnea
While CPAP machines are the most commonly prescribed treatment for sleep apnea, there are several other therapies available to those who cannot or choose not to use CPAP. Positional therapy, oral appliance therapy, and lifestyle modifications can all be effective treatments for mild-to-moderate sleep apnea.
Positional Therapy
Positional therapy involves sleeping on your side or stomach instead of on your back, a common cause of obstructive sleep apnea. To do this safely and effectively, you may need to elevate the head of your bed by 4-6 inches (10-15 cm). Alternatively, a device that prevents you from turning onto your back during sleep can be used.
Oral Appliance Therapy
Oral Appliance Therapy is indicated for mild to moderate obstructive sleep apnea and involves wearing a dental device, that looks like two mouthguards joined with connectors, that are designed to support and position the jaw so that airways remain open during sleep.
Surgical Options
There are several surgical procedures available for those with moderate-to-severe sleep apnea. These include removing sinus tissue, realigning the jaw or both upper and lower jaws (maxillomandibular advancement), or performing a tracheostomy.
Lifestyle Changes
Making lifestyle changes (such as losing weight, avoiding alcohol and smoking) can be effective in reducing the symptoms of mild-to-moderate sleep apnea
The Impact on Sleep Apnea Patients
While the recall process has been relatively smooth for some patients, for others, it has been a source of significant disruption and concern. For those who rely on their CPAP machines to treat severe obstructive sleep apnea and other sleep disorders, the prospect of returning their device or finding a suitable replacement can be anxiety-provoking.
One of the biggest concerns for affected patients has been the potential disruption to their treatment.
While Philips Respironics has offered to repair or replace recalled machines at no cost, the process of returning the device and waiting for a replacement can take time. This can be especially concerning for those who use their CPAP machines every night and may struggle to sleep without them.
Another concern for affected patients has been finding a suitable replacement CPAP machine. For some, the DreamStation or System One model that they were using was the only device that they have ever used, and the prospect of switching to a different model or brand can be daunting.
While Philips Respironics has assured customers that they will do their best to provide a replacement machine that meets the patient’s needs, the process of finding a new device that is comfortable and effective can be challenging for some.
Overall, the Philips Respironics CPAP recall has been a source of disruption and concern, in the sleep field. While the company has taken steps to address the issue and offer repairs or replacements at no cost, the process of returning or finding a new device can be stressful for those who rely on their CPAP machines to assure quality sleep and use them every night.
Affected patients need to stay in contact with Philips Respironics and their sleep specialist or DME (durable medical equipment) provider to ensure that their treatment is not disrupted and that they can find a suitable replacement device.
Conclusion
The voluntary recall of certain models of Philips Respironics CPAP machines due to potential health risks associated with exposure to polyester-based polyurethane (PE-PUR)foam, has been a source of disruption and concern for some sleep apnea patients.
However, it is important to remember that the recall was implemented as a proactive measure to ensure the safety of the devices and to prevent any potential harm to patients.
Philips Respironics has taken steps to address the recall by setting up a dedicated recall website and hotline, and by directly contacting affected customers with instructions on how to proceed.
The company has also offered to repair or replace recalled machines at no cost, to minimize the disruption to patients’ treatment.
While the process of returning or finding a new CPAP machine can be anxiety-provoking for some.
It is important to stay informed about product recalls and follow the instructions provided by the manufacturer to ensure the safety of any medical device, not just CPAP.
By staying informed and working with your sleep specialist or DME (durable medical equipment) provider, you can ensure that your treatment is not disrupted and that you have access to a safe and effective device.